Hello friends. Hopefully we all learned recently that higher than expected levels of fluoride are common in our municipal water supplies. “The report, based on an analysis of previously published research, marks the first time a federal agency has determined — “with moderate confidence” — that there is a link between higher levels of fluoride exposure and lower IQ in kids.” [1]
The most concerning admission is that years of clinical data shows the IQ destroying amount of fluoride is only double the allowed amount. We also learn that regulators have steadily increased the threshold for dosing. “Since 2015, federal health officials have recommended a fluoridation level of 0.7 milligrams per liter of water, and for five decades before the recommended upper range was 1.2. The World Health Organization has set a safe limit for fluoride in drinking water of 1.5.” [1]
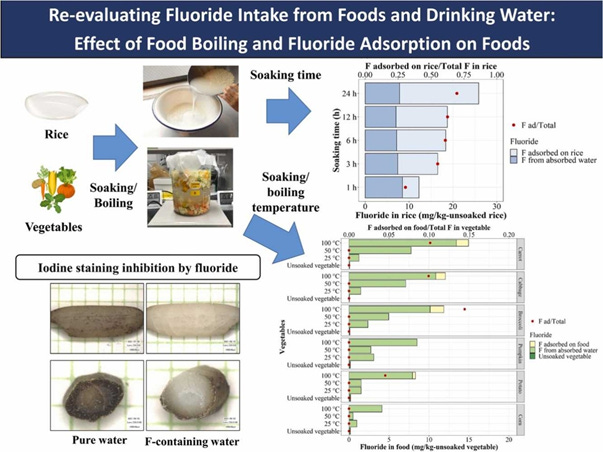
That doesn’t seem scary and is appropriate dosing until we factor in daily use case scenarios. The most common example is boiling water which dramatically increases concentration. From this 2023 paper “Re-evaluating fluoride intake from food and drinking water: Effect of boiling and fluoride adsorption on food” we learn these shocking details:
“a. Rice soaked in water accumulates fluoride by absorption and adsorption.
b. Boiling food in fluoride-containing water highly increases fluoride content in food.
c. Fluoride interferes with the iodine staining of starch contained in rice.
d. Infants ingest more fluoride per body weight than do children and adults.” [2]
Sadly, we see, fluorides affinity to plant calcium and heat as ideal scenarios for “absorption and adsorption” to vulnerable populations that would include babies, elderly and the immunocompromised which will be covered in later parts of this series.
Lets take a moment to understand these two ways fluoride overwhelms the bodies ability to detoxify it. Firstly absorption “Absorption is a physical or chemical phenomenon or a process in which atoms, molecules or ions enter the liquid or solid bulk phase of a material. This is a different process from adsorption, since molecules undergoing absorption are taken up by the volume.” [3]
Secondly, and much more important to the hair follicle disaster is adsorption of the fluoride ion onto the sebaceous glands that will be discussed in the coming parts. “Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid (the absorbate) is dissolved by or permeates a liquid or solid (the absorbent).
While adsorption does often precede absorption, which involves the transfer of the absorbate into the volume of the absorbent material, alternatively, adsorption is distinctly a surface phenomenon, wherein the adsorbate does not penetrate through the material surface and into the bulk of the adsorbent. The term sorption encompasses both adsorption and absorption, and desorption is the reverse of sorption.
Like surface tension, adsorption is a consequence of surface energy. In a bulk material, all the bonding requirements (be they ionic, covalent or metallic) of the constituent atoms of the material are fulfilled by other atoms in the material. However, atoms on the surface of the adsorbent are not wholly surrounded by other adsorbent atoms and therefore can attract adsorbates. The exact nature of the bonding depends on the details of the species involved, but the adsorption process is generally classified as physisorption (characteristic of weak van der Waals forces) or chemisorption (characteristic of covalent bonding). It may also occur due to electrostatic attraction. The nature of the adsorption can affect the structure of the adsorbed species. For example, polymer physisorption from solution can result in squashed structures on a surface.
Adsorption is present in many natural, physical, biological and chemical systems and is widely used in industrial applications such as heterogeneous catalysts, activated charcoal, capturing and using waste heat to provide cold water for air conditioning and other process requirements (adsorption chillers), synthetic resins, increasing storage capacity of carbide-derived carbons and water purification. Adsorption, ion exchange and chromatography are sorption processes in which certain adsorbates are selectively transferred from the fluid phase to the surface of insoluble, rigid particles suspended in a vessel or packed in a column. Pharmaceutical industry applications, which use adsorption as a means to prolong neurological exposure to specific drugs or parts thereof,[citation needed] are lesser known.” [4]
So with these two forms of bonding in mind let’s look at first understanding the actual evidence to the Hair Follicle Disaster that we can now confirm.
“Foods, namely rice and vegetables, accumulate fluoride when soaked
in fluoride-containing water. Rice accumulates fluoride in two ways:
absorption and adsorption. In this study, boiling foods at 100 ◦C
significantly increased the fluoride content in rice and vegetables.
Therefore, fluoride intake from food could be a major source of fluoride
intake if fluoride-containing water is used for soaking and boiling food.
Thus, groups with low body weights, namely infants and children, are
likely to intake high amounts of fluoride per body weight. Even though a
fluoride concentration in drinking water of 1.5 mg/L is considered safe,
assuming that the fluoride intake from food is 0.01 mg/kg bw/d, our
study indicates that this intake can be higher than previous estimations
(at 0.012–0.023 mg/kg bw/d) due to the accumulation of fluoride in
foods cooked with fluoride-containing water. This study proved that the
fluoride intake ratio from food to water could be higher than 0.20
depending on the food, cooking methods, and cooking water.” [2]
Wow right? Many have speculated in the past that this was the case, but this research concluded that the suspicions are true, and with solid evidence showing how our children are not only at risk but are being damaged regularly by overexposure to fluoride from normal cooking and bathing habits.
[1] “US government report says fluoride at twice the recommended limit is linked to lower IQ in kids”
https://www.msn.com/en-us/health/other/us-government-report-says-fluoride-at-twice-the-recommended-limit-is-linked-to-lower-iq-in-kids/ar-AA1pcF6o
[2] “Re-evaluating fluoride intake from food and drinking water: Effect of boiling and fluoride adsorption on food”
https://www.sciencedirect.com/science/article/pii/S0304389422019562
[3] https://en.wikipedia.org/wiki/Absorption_(chemistry)
[4] https://en.wikipedia.org/wiki/Adsorption




elucidating.
What is it that would combine with Fluoride and Chloride ions to deposit them out of the water without adding further issues?
Would these also, then, interfere with the metabolism of the essential iodine?
Would these be an issue with the thyroid?